Products
Salofalk
Salofalk (mesalazine) is used to treat active UC and maintain patients in remission and is available in 2 oral and 3 rectal formulations:
- For oral administration
- Salofalk granules – prolonged release formulation available in 3g, 1.5g, 1g and 500mg sachets
- Salofalk tablets – gastro-resistant formulation available as 1g, 500mg and 250mg tablets
- For rectal administration
- Salofalk 2g liquid enema (4g enema, Ireland)
- Salofalk 1g rectal foam
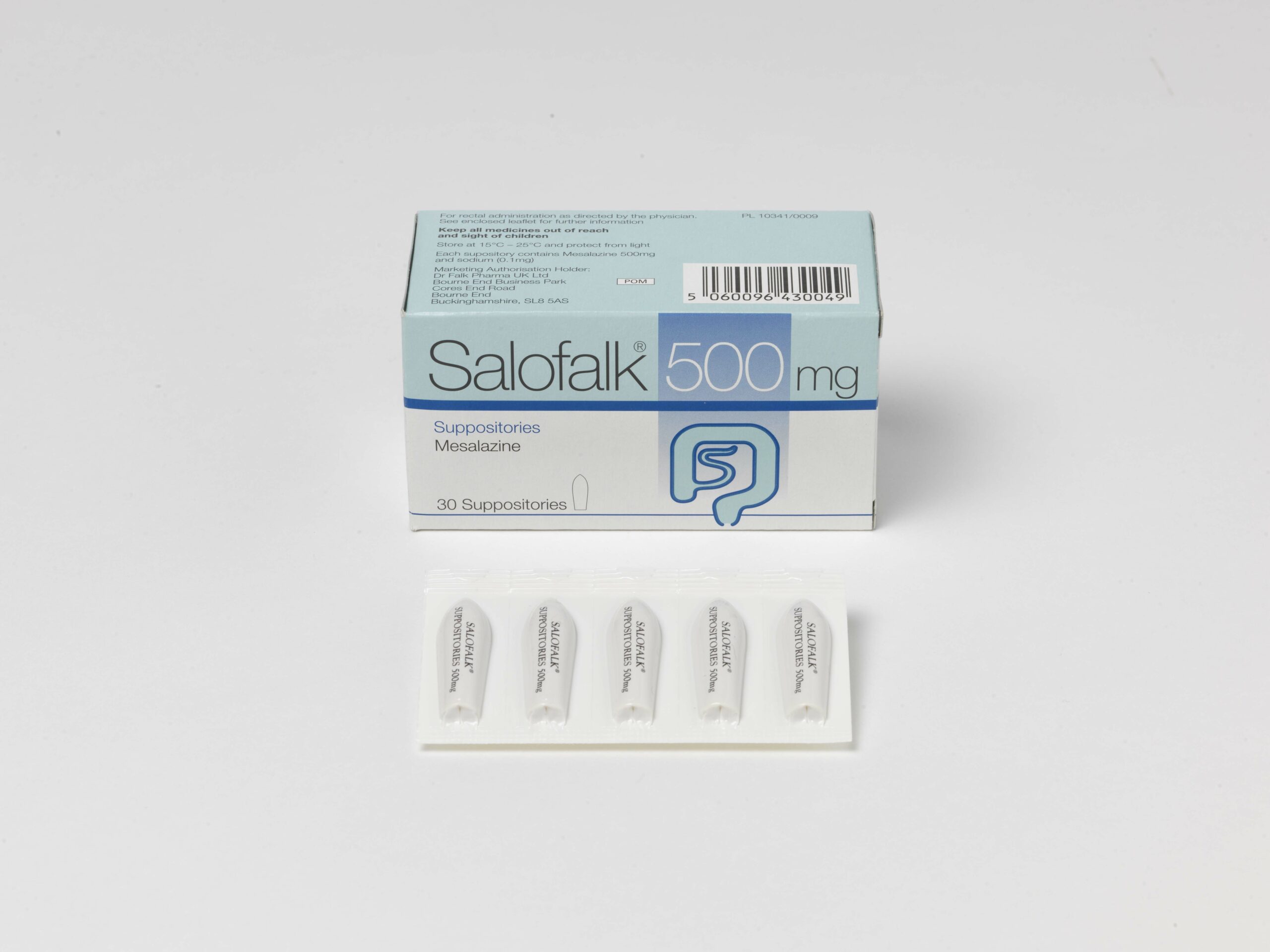
- Salofalk 500mg and 1g suppositories (250mg suppositories, Ireland)

Discover more about Salofalk
Research shows the location, extent and severity of the disease varies from patient to patient. Therefore, effective treatment of ulcerative colitis throughout the colon requires a range of product formulations to provide individual and targeted patient treatment.
Prescribing Information (refer to full SPC before prescribing):
Presentation: Salofalk 250mg gastro-resistant tablets: gastro-resistant tablet containing 250mg mesalazine. Salofalk 500mg and 1g gastro-resistant tablet (UK ONLY) containing 500mg and 1g mesalazine respectively. Salofalk 500mg/1000mg/1500mg/3000mg prolonged-release granules: prolonged-release granules containing 500mg,1000mg, 1500mg or 3000mg mesalazine per sachet. Indications: Salofalk 250mg tablets (UK): treatment and maintenance of remission of mild/moderate ulcerative colitis. Salofalk 250mg tablets (Ireland): as an anti-inflammatory in the management of ulcerative colitis and in the treatment of Crohn’s disease. Salofalk 500mg tablets (UK): treatment and maintenance of remission of ulcerative colitis. Salofalk 1g tablets (UK): treatment of acute episodes of mild/moderate ulcerative colitis. Salofalk granules: treatment of acute episodes and the maintenance of remission of mild to moderate ulcerative colitis. Dosage: Salofalk 250mg tablets – Adults and elderly: acute treatment 6 -12 tablets daily in 3 divided doses. Maintenance treatment 6 tablets daily in 3 divided doses. Salofalk 500mg tablets (UK only): 1 or 2 tablets 3 times daily. Maintenance: 1 tablet 3 times daily. Salofalk 1g tablets (UK only): 1 tablet three times daily. Salofalk granules – adults: acute treatment – once daily 1 sachet of 3g granules, 1 or 2 sachets of Salofalk 1.5g granules, 3 sachets of 500mg granules or 3 sachets of 1000mg granules (equivalent to 1.5 – 3.0g mesalazine daily), preferably taken in the morning. Alternatively, the dose can be taken divided in three doses. Maintenance treatment – 1 sachet of 500mg granules 3 times daily (1.5g mesalazine daily). Where needed, 3.0g per day in a single morning dose may be taken. Method of administration: oral. Tablets – taken whole without chewing, with liquid, one hour before meals. Granules – taken on the tongue and swallowed, without chewing, with plenty of liquid. Duration of treatment is usually 8 weeks. To be determined by physician. Children (all formulations): there is only limited documentation for an effect in children (age 6-18 years). Children 6 years and older: active disease – on individual basis starting with 30-50mg/kg/day either once daily (granules) or in divided doses (tablets and granules). Maximum 75mg/kg/day. Total dose should not exceed recommended adult dose. Maintenance – on individual basis starting with 15-30mg/kg/day in divided doses. Total dose should not exceed recommended adult dose. Generally recommended that half the adult dose may be given to children up to a body weight of 40kg and the normal adult dose to those above 40kg. Contra-indications: Hypersensitivity to salicylates or any of the excipients. Severe impairment of renal or hepatic function. Warnings/Precautions: Blood tests and urinary status (dip sticks) should be determined prior to and during treatment. Caution is recommended in patients with impaired hepatic function. Should not be used in patients with impaired renal function. Mesalazine-induced renal toxicity should be considered if renal function deteriorates during treatment – stop treatment immediately in such cases. Cases of nephrolithiasis reported; ensure good hydration. Serious blood dyscrasias have been reported very rarely with mesalazine. Hematological investigations should be performed if patients suffer from unexplained haemorrhages, bruises, purpura, anaemia, fever or pharyngolaryngeal pain. Salofalk should be discontinued in case of suspected or confirmed blood dyscrasia. Cardiac hypersensitivity reactions (myocarditis, and pericarditis) induced by mesalazine have been rarely reported. Salofalk should then be discontinued immediately. Patients with pulmonary disease, in particular asthma, should be carefully monitored. Severe cutaneous adverse reactions (SCARs), including drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported. Discontinue treatment at the first appearance of signs and symptoms of severe skin reactions, such as skin rash, mucosal lesions, or any other sign of hypersensitivity. Patients with a history of adverse drug reactions to preparations containing sulphasalazine should be kept under close medical surveillance. If acute intolerance reactions e.g., abdominal cramps, acute abdominal pain, fever, severe headache and rash occur, stop treatment immediately. Tablets
may be excreted undissolved in patients with the ileocecal valve removed. Salofalk granules: contain aspartame, a source of phenylalanine. May be harmful to patients with phenylketonuria. Granules also contain sucrose: 0.04mg, 0.08mg, 0.12mg, 0.24mg (500mg/1g/1.5g and 3g granules respectively). Salofalk tablets: For patients on a sodium-controlled diet: the 250mg and 500mg tablets contain 48mg and 49mg of sodium, equivalent to 2.4% and 2.5% of the recommended maximum daily intake for sodium. Urine may be discoloured red-brown after contact with sodium hypochlorite bleach used in toilets. Interactions: Specific interaction studies have not been performed. With concomitant treatment with azathioprine, 6-mercaptopurine or thioguanine, consider a possible increase in their myelosuppressive effects. There is weak evidence that mesalazine might decrease the anticoagulant effect of warfarin. Salofalk granules (additionally): lactulose, or similar preparations which lower stool pH: possible reduction of mesalazine release from granules due to decreased pH caused by bacterial metabolism of lactulose. Use in pregnancy and lactation: do not use Salofalk during pregnancy unless the potential benefit outweighs the possible risks. Limited experience in the lactation period. Salofalk should only be used during breast-feeding if the potential benefit outweighs the possible risks; if the breast-fed infant develops diarrhoea, breast-feeding should be discontinued. Undesirable effects: altered blood counts (aplastic anaemia, agranulocytosis, pancytopenia, neutropenia, leukopenia, thrombocytopenia), hypersensitivity reactions such as allergic exanthema, drug fever, lupus erythematosus syndrome, pancolitis, headache, dizziness, peripheral neuropathy, peri- and myo-carditis, allergic and fibrotic lung reactions (including dyspnoea, cough, bronchospasm, alveolitis, pulmonary eosinophilia, lung infiltration, pneumonitis), abdominal pain, diarrhoea, dyspepsia, flatulence, nausea, vomiting, acute pancreatitis, cholestatic hepatitis, hepatitis, rash, pruritus, photosensitivity – especially with pre-existing skin conditions, alopecia, severe cutaneous adverse reactions (SCARs) including drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), arthralgia, myalgia, impairment of renal function including acute and chronic interstitial nephritis and renal insufficiency, nephrolithiasis, asthenia, fatigue, oligospermia (reversible), changes in hepatic function parameters, changes in pancreatic enzymes, eosinophil count increased. Legal category: POM. Cost (UK – basic NHS price; Ireland – PtW): Salofalk 250mg tablets (100s) £16.19; €13.48. Salofalk 500mg tablets (100s) £32.38. Salofalk 1g tablets (90s) £58.50. Salofalk 500mg granules (100 sachets) £28.74; €27.93. Salofalk 1000mg granules (50 sachets) £28.74; €32.87. Salofalk 1500mg granules (60 sachets) £48.85; €49.66. Salofalk 3g granules (60 sachets) £97.70; €99.73. Product licence number: Salofalk 250mg tablets: PL10341/0004; PA573/4/3. Salofalk 500mg tablets: PL08637/0019. Salofalk 1g tablets: PL08637/0027. Salofalk 500mg granules: PL08637/0007; PA573/3/1. Salofalk 1000mg granules: PL08637/0008; PA573/3/2. Salofalk 1500mg granules: PL08637/0016; PA573/3/7. Salofalk 3g granules: PL08637/0025; PA573/3/6. Product licence holder: Salofalk 250mg tablets in the UK: Dr Falk Pharma UK Ltd, Bourne End Business Park, Cores End Road, Bourne End, SL8 5AS. Salofalk 500mg and 1g tablets and all granules: Dr Falk Pharma GmbH, Leinenweberstr.5, D-79108 Freiburg, Germany. Date of preparation: February 2024
Further information is available on request.
Adverse events should be reported. In the UK: Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ In Ireland: Reporting forms and information can be found at https://www.hpra.ie/homepage/about-us/report-an-issue/human-adverse-reaction-form Adverse events should also be reported to Dr Falk Pharma UK Ltd at PV@drfalkpharma.co.uk.
Prescribing Information (Please refer to full SPC before prescribing)
Presentation: Salofalk® 1g/actuation rectal foam – containing 1g mesalazine per actuation. Salofalk® enema 2g – enema containing 2g mesalazine in 59ml of suspension. Salofalk® 4g/60ml enema – enema containing 4g mesalazine in 60ml of suspension. Salofalk® 250mg Suppositories – suppositories containing 250mg mesalazine. Salofalk® Suppositories 500mg – suppositories containing 500mg mesalazine. Salofalk® 1g Suppositories – suppositories containing 1g mesalazine. Indications: Salofalk 1g rectal foam: treatment of active, mild ulcerative colitis of the sigmoid colon and rectum. Salofalk enema 2g: treatment and prophylaxis of acute attacks of mild ulcerative colitis, especially in the rectum and sigmoid colon and also in the descending colon. Salofalk 4g/60ml enema and 250mg suppositories: as an anti-inflammatory in the management of ulcerative colitis, alone, or, particularly in the acute phase, with corticosteroids. Salofalk 500mg and 1g suppositories: treatment of mild and moderate attacks of ulcerative colitis, in the rectum. Dosage: Salofalk 1g Rectal Foam – adults: 2 administrations once a day at bedtime. Use at room temperature. 1 administration twice a day is possible. Salofalk 2g and 4g enema – adults and elderly: 1 enema a day at bedtime. Salofalk 250mg suppositories – adults and elderly: 2 suppositories rectally 3 times daily. In severe cases of the disease, the dosage may be doubled. For long term treatment and prevention of recurrences, one suppository three times daily. Salofalk suppositories 500mg – adults and elderly: 1–2 suppositories, 2–3 times daily; Salofalk 1g suppositories – adults and elderly: 1 suppository once daily preferably at bedtime. Do not discontinue treatment suddenly. Duration of treatment – to be determined by physician. All formulations: children: there is little experience and only limited documentation for an effect in children. Contra-indications: known hypersensitivity to salicylates or any of the excipients. Severe impairment of hepatic or renal function. The sulphite component of Salofalk foam may cause hypersensitivity reactions in patients with asthma. Warnings/precautions: blood tests and urinary status should be determined prior to and during treatment. Caution recommended in patients with impaired hepatic function. Not to be used in patients with impaired renal function. Consider mesalazine-induced renal toxicity if renal function deteriorates during treatment. Discontinue treatment immediately. Cases of nephrolithiasis reported; ensure good hydration. Urine may be discoloured red-brown after contact with sodium hypochlorite bleach used in toilets. Serious blood dyscrasias have been reported very rarely with mesalazine. Hematological investigations should be performed if patients suffer from unexplained haemorrhages, bruises, purpura, anaemia, fever or pharyngolaryngeal pain. Salofalk should be discontinued in case of suspected or confirmed blood dyscrasia. Cardiac hypersensitivity reactions (myocarditis and pericarditis) induced by mesalazine have been rarely reported. Salofalk should then be discontinued immediately. Monitor patients with pulmonary disease especially asthma. Severe cutaneous adverse reactions (SCARs), including drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported. Discontinue treatment at the first appearance of signs and symptoms of severe skin reactions, such as skin rash, mucosal lesions, or any other sign of hypersensitivity. Keep patients with a history of adverse drug reactions to preparations containing sulphasalazine under close medical surveillance. Discontinue treatment if Salofalk causes acute intolerance reactions such as abdominal cramps, acute abdominal pain, fever, severe headache and rash. Special notes: Salofalk foam: propylene glycol may cause skin irritation, sodium metabisulphite may rarely cause severe hypersensitivity reactions and bronchospasm, cetostearyl alcohol may cause local skin reactions. Salofalk 2g and 4g enemas: potassium metabisulphite may rarely cause severe hypersensitivity reactions and bronchospasm. Sodium benzoate may cause local irritation. Salofalk 500mg suppositories: cetyl alcohol may cause local skin reactions. Interactions: specific interaction studies have not been performed. In patients concomitantly treated with azathioprine, 6-mercaptopurine or thioguanine, an increase in their myelosuppressive effects should be taken into account. There is weak evidence that mesalazine might decrease the anticoagulant effect of warfarin. Use in pregnancy and lactation: there are no adequate data on use in pregnant women. Only use during pregnancy if the potential benefit outweighs the possible risk. Acetylated mesalazine passes into breast milk. Only use during breast-feeding if the potential benefit outweighs the possible risk. If the infant develops diarrhoea breast-feeding should be discontinued. Undesirable effects: rash, pruritus, headache, dizziness, peri- and myocarditis, abdominal pain, diarrhoea, flatulence, nausea, vomiting, constipation (except foam), photosensitivity especially with pre-existing skin conditions, altered blood counts (aplastic anaemia, agranulocytosis, pancytopenia, neutropenia, leukopenia, thrombocytopenia), peripheral neuropathy, allergic and fibrotic lung reactions (including dyspnoea, cough, bronchospasm, alveolitis, pulmonary eosinophilia, lung infiltration, pneumonitis), acute pancreatitis, impairment of renal function including acute and chronic interstitial nephritis and renal insufficiency, alopecia, myalgia, arthralgia, hypersensitivity reactions such as allergic exanthema, drug fever, lupus erythematosus syndrome, pancolitis, changes in hepatic function parameters, hepatitis, cholestatic hepatitis, oligospermia (reversible), nephrolithiasis, severe cutaneous adverse reactions (SCARs) including drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN). Salofalk rectal foam may also cause abdominal distension, anal discomfort, application site irritation and painful rectal tenesmus. Legal category: POM. Basic UK NHS cost and Irish price to wholesaler: Salofalk 1g/actuation rectal foam, 14 administrations per container – £30.17; €31.55. Salofalk enema 2g, 7 enemas – £29.92; Salofalk 4g/60ml enema, 7 enemas – €30.36. Salofalk 250mg suppositories, 30 suppositories – €10.70; Salofalk suppositories 500mg, 30 suppositories – £14.81. Salofalk 1g suppositories, 30 suppositories – £29.62; €36.49. Product licence number: Salofalk 1g/actuation rectal foam – PL08637/0003; PA 573/4/5. Salofalk enema 2g – PL10341/0008. Salofalk 4g/6ml enema – PA 573/4/1. Salofalk 250mg suppositories – PA 573/4/2. Salofalk suppositories 500 mg – PL10341/0009. Salofalk 1g Suppositories – PL08637/0018; PA573/4/4. Product licence holder: Salofalk 1g rectal foam, Salofalk 4g/60ml enema, Salofalk 250mg and 1g Suppositories: Dr Falk Pharma GmbH, Leinenweberstr. 5, D-79108 Freiburg, Germany. Salofalk enema 2g, Salofalk suppositories 500mg: Dr Falk Pharma UK Limited, Unit K, Bourne End Business Park, Cores End Road, Bourne End, SL8 5AS, United Kingdom. Date of preparation: September 2023.
Further information is available on request.
Adverse Events should be reported
Ireland:
Reporting forms and information can be found at https://www.hpra.ie/homepage/about-us/report-an-issue/human-adverse-reaction-form
United Kingdom:
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.co.uk
Adverse events should also be reported to Dr Falk Pharma UK Ltd at PV@drfalkpharma.co.uk
Summary of Product Characteristics (SmPC)
UK
For further information, the Summary of Product Characteristics (SmPC) are available below.
- Salofalk 0.5g Prolonged Release Granules SmPC
- Salofalk 1g Prolonged Release Granules SmPC
- Salofalk 1.5g Prolonged Release Granules SmPC
- Salofalk 3g Prolonged Release Granules SmPC
- Salofalk 250mg Gastro-resistant Tablets SmPC
- Salofalk 500mg gastro-resistant Tablets SmPC
- Salofalk 1g Tablets SmPC
- Salofalk 2g Enema SmPC
- Salofalk 1g/actuation Rectal Foam SmPC
- Salofalk 500mg Suppositories SmPC
- Salofalk 1g Suppositories SmPC
Republic of Ireland
For further information, the Summary of Product Characteristics (SmPC) are available below.
- Salofalk 0.5g Prolonged Release Granules SmPC
- Salofalk 1g Prolonged Release Granules SmPC
- Salofalk 1.5g Prolonged Release Granules SmPC
- Salofalk 3g Prolonged Release Granules SmPC
- Salofalk 250mg Gastro-resistant Tablets SmPC
- Salofalk 4g Enema SmPC
- Salofalk 1g/actuation Rectal Foam SmPC
- Salofalk 250mg Suppositories SmPC
- Salofalk 1g Suppositories SmPC
Patient Resources
This information on the following pages is intended only for UK and Irish residents who have been prescribed either Budenofalk, Salofalk, Ursofalk or Jorveza.

